The World Health Organization said Tuesday it has granted emergency use of a Japanese mpox vaccine, its second approval of a product to inoculate against the infectious disease, to combat a recent outbreak mainly in Africa.
The approval of the vaccine, developed by KM Biologics Co., a group firm of food and pharmaceutical company Meiji Holdings Co., follows the acceptance of a vaccine manufactured by Bavarian Nordic A/S of Denmark in September.

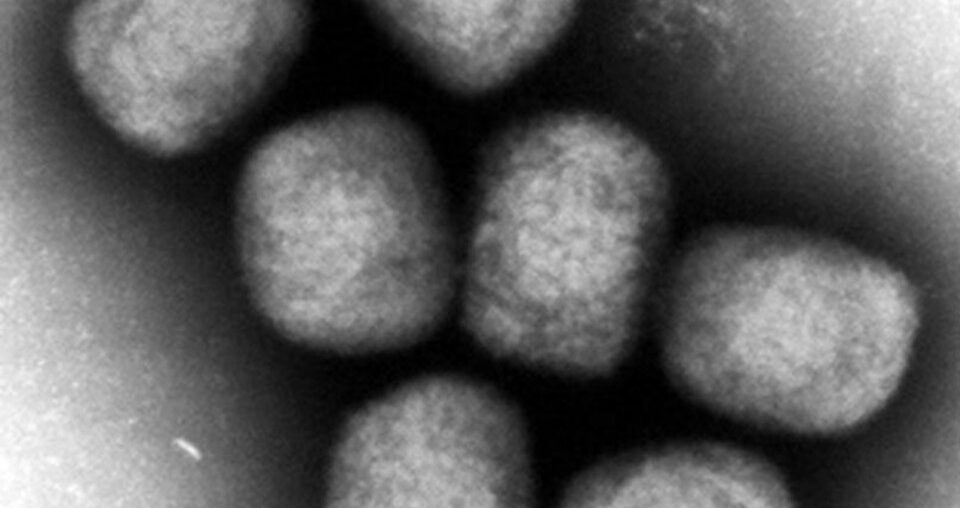
An electron microscopic image shows mpox virus particles.(Image courtesy of Japan’s National Institute of Infectious Diseases)(Kyodo)
“This decision is expected to facilitate increased and timely access to vaccines in communities where mpox outbreaks are surging,” the Geneva-based U.N. health body said in a statement.
The vaccine, which was developed for smallpox, has been approved in Japan as also effective for mpox. The country applied for its emergency use with the WHO in August.
The WHO declared in August that an alarming rise in mpox cases in the Democratic Republic of the Congo and neighboring countries constitutes a public health emergency of international concern, a designation previously used for COVID-19.
Japan pledged to donate 3.05 million doses of the vaccine, along with specialized inoculation needles, to the Democratic Republic of the Congo, according to the WHO.
In 2024, cases of mpox have been reported in 80 countries, including 19 in Africa, the WHO said. The Democratic Republic of the Congo recorded over 39,000 suspected cases and 1,000 deaths, it said.